Simply being born in other countries and in different communities defines and limits what kind of a woman a girl can become, thereby programming her future life.
FIGIJ aims to reduce cervical cancer in Africa through human papilloma virus vaccination (HPV)
Young people cannot advocate for themselves, and deserve the very best chance at a healthful life. Therefore, FIGIJ stimulates, motivates and educates PAG experts from all corners of the globe to serve this population of young people and to advocate for the health of our youth worldwide. This has been reflected in the wonderful ImaGYNations project, recently launched by the FIGIJ Board. ImaGYNations is a political call for unified efforts and commitments, to make the world a better place for girls, boys, young men and women in need.
FIGIJ helps children and adolescents to access modern and safe family planning methods, which eventually promotes girls and young women to become eventual leaders in politics, peace processes and public life by removing the barriers that so often prevent them from contributing to and benefitting from health development. Education and family planning for young girls are also identified among the top 100 solutions for reversing global warming.
In recent years, the awareness of HPV transmission has created much concern among health-care providers in Western countries. This is expressed through the constant attention towards HPV vaccination from international health and human rights organizations as well as from the world media.
There is a raging debate regarding the position of Western societies.
The World Health Organization (WHO) estimates that HPV related cervical cancer is a leading cause of death worldwide, but the inconvenient truth is that the need for action is underestimated. Here lies the controversy as to whether each individual has a right to preventive medicine.
Seeing thousands of communities all over the world, it has become a pleasure and a duty to give anyone an equal chance to live healthy.
The ambitious project to end HPV related cancer is brilliantly led by multiple organisations. Slowly but surely things are changing for the better.
Women and girls and soon many men and boys, will get the available vaccination that reduces the HPV related disease.
Unfortunately, less and undeveloped countries are left behind.
The aim of our ImaGYNations call for action is to provide a solution for a complex issue.
FIGIJ hopes to support and accelerate the pace of change which might result in a reduction of these HPV virus related cancers.
Cultural influences can change the perception of usefulness of HPV vaccination policy as well as its social acceptance. Social stigmatisation can have a negative impact and with respect for other cultures, approach should be tailor made. Vaccination asks for proper counseling of the targeted population.
- Prevention of cervical cancer cases in vulnerable populations.
- Single dose HPV vaccination to one million children within designated African countries in the first wave of the programme to be completed over the next three years.
- Anticipated impact in 20-25 years
- The International Federation of Pediatric and Adolescent Gynecology (FIGIJ)
- The International Centre for Reproductive Health (ICRH)
FIGIJ is now developing a worldwide PAG education strategy and supports preventive HPV prophylactic vaccination triggered by the knowledge (Van Damme et al) that the safety period obtained by the implementation is as long as >30 years.
This project offers an opportunity for partners to engage in global research opportunities in partnership with ImaGYNations including the WHO allowing for an opportunity to review their Guidelines on HPV vaccination.
________________________________________
Background
Cancer kills more people worldwide than AIDS, tuberculosis, and malaria together. Cervical cancer is the fourth most common cancer in women with 528,0000 new cases diagnosed worldwide in 2012. Death from cervical cancer is typically protracted, painful and unpleasant. Enormous efforts have been made to allow for prevention, early diagnosis and treatment. National screening programmes having developed exacting standards of care for cytology screening, colposcopy, treatment of pre-cancerous changes, multidisciplinary networks for the management of cervical cancer and management protocols involving surgical and adjunctive therapies. All of this requires intensive investment and infrastructure and works well where such resources are available. Invariably these resources are not available in Africa where alternative strategies could and should be considered in order to prevent cervical cancer from occurring in the first place. Cervical cancer is the second most common cancer among women in Africa, exacerbated by the lack of reproductive health information for women and delayed access to treatment in rural areas. These women often present in an advanced state of the disease. In Africa where screening opportunity is low, treatment remains mostly inaccessible and cervical cancer is considered being a deadly disease.
By introducing a system of prevention based widespread single dose human papilloma virus vaccination it is proposed to reduce the incidence of cervical cancer in African women.
The relevance and impact on HPV vaccination on reducing the prevalence of cervical cancer is now recognised, so that 58 countries including many European countries have established defined universal programmes for HPV vaccination in adolescent females. The figure below illustrates the extent to which universal vaccination programmes were in place in Europe in 2015.
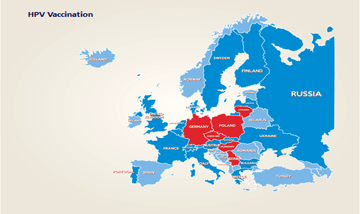
Dark blue – National HPV vaccination programme
Red – No National HPV vaccination programme in place
Pale Blue – No response
By way of example, the European Board and College of Obstetrics and Gynaecology published a series of Gynaecological Standards of Care for Women’s Health in Europe in May 2014 (www.ebcog.eu). The section headed Paediatric and Adolescent Gynaecology includes the statement that vaccination against Human Papilloma Virus (HPV) should be promoted and offered to all adolescents and that national prevention programmes should be encouraged. No such national prevention programmes exist in Africa where globally there are the highest rates of this condition. Focus on promotion is needed, as dis and lack of knowledge leads to mistrust and low uptake. Convenient delivery platforms must be chosen, and school-based vaccination remains a good option.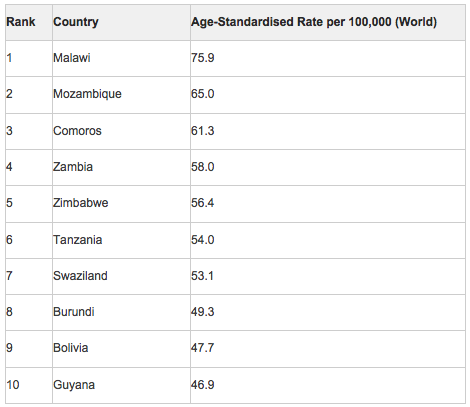
Approximately 84% of cervical cancer cases occur in less developed countries. The highest incidence of cervical cancer is in Africa, Latin America and the Caribbean. Lowest rates were in North America and Oceania, with lowest rates of 7-9/100000 achieved in countries with screening programmes including the UK, Australia and New Zealand and North America.
The countries with the top 20 highest incidence of cervical cancer in 2012 include Malawi followed by Mozambique and Comoros.
Vignette – Malawi
 With sustained high fertility in the last 20 years, the age structure of Malawi’s population is extremely youthful. Two-thirds of the population is under the age of 25, placing a significant burden on the working-age population to provide the basic health and education needs required by children and youth. Proper investments in the well-being of children and adolescents will help ensure that current and future generations will grow and develop into their full potential.
With sustained high fertility in the last 20 years, the age structure of Malawi’s population is extremely youthful. Two-thirds of the population is under the age of 25, placing a significant burden on the working-age population to provide the basic health and education needs required by children and youth. Proper investments in the well-being of children and adolescents will help ensure that current and future generations will grow and develop into their full potential.
Completion of secondary education is low among young adults ages 20-24, particularly for girls. Those in the wealthiest income level have the highest completion rates. Students who stay in school longer tend to delay marriage, have smaller families and more economic opportunities, and are better informed about health-related behaviours.
Among employed adolescents aged 15-18 years, two thirds work in the agricultural sector; 17% work in manual labour; and 14% work in sales. When adolescents enter the labour force, they are often unable to continue their education, preventing them from building the skills necessary for meaningful employment.
Adolescent girls aged 15-19 years are 10 times more likely to be married than adolescent boys. Early marriage puts young girls at risk of early childbearing and birth complications, prevents them from completing school, and limits their economic opportunities.
More than 1 in 4 adolescent boys have had sex before the age of 15 years, twice as many as adolescent girls. Providing family planning to young people reduces risk of disease and unintended pregnancy, and promotes a healthy transition to adulthood. Given the high prevalence of HIV, it is important to reach young people with information about how to avoid infection.
In Malawi, a child is legally defined as any person under the age of 18 years. More than 1 in 5 adolescent girls have begun bearing children by the age of 17 years. Early childbearing is a major health concern because of the increased risks of death and disability to both mother and child during pregnancy and childbirth.
The Malawi Demographic and Health Survey 2015-16 Key Indicators Report published in May 2016 has identified that 29% of adolescents aged 15-19 years in Malawi have begun childbearing: 22% of women aged 15-19 years have given birth, and another 7% were pregnant with their first child at the time of interview.
As expected, the proportion of women aged 15-19 years who have begun childbearing rises rapidly with age, from 5% among women aged 15 years, to 27% among women aged 17 years, and 59% at 19 years of age. Early childbearing among teenagers is more common in rural than in urban areas (31% versus 21% respectively) and among women in the northern and southern regions (32% each) compared with the central region (25%).
The proportion of teenagers who have started childbearing decreases with increasing level of education: more than half of teenagers aged 15-19 years with no education (54%) have begun childbearing compared with 32% of teenagers who have attained primary education and 19% of those who have attained the secondary education.
Teenagers in the lowest wealth quintile tend to start childbearing earlier than those in the highest quintile (44% versus 15%, respectively).
Universal immunisation of children against six common vaccine-preventable diseases, namely tuberculosis, diphtheria, whooping cough (pertussis), tetanus, polio, and measles, is employed to reduce infant and child mortality. Other childhood vaccines given in Malawi protect against hepatitis B and Haemophilus influenzae type b (Hib). The government of Malawi introduced the pneumococcal conjugate vaccine (PCV 13) and monovalent human rotavirus vaccine (RV1) into the national’s infant immunisation programme in November 2011 and October 2012, respectively. The pneumococcal vaccine protects against Streptococcus pneumoniae bacteria, which cause severe pneumonia, meningitis, and other illnesses. Rotavirus is a virus that causes gastroenteritis; an inflammation of the stomach and intestines. If left untreated, it can lead to severe dehydration and death.
The 2015-16 survey collected information on the coverage of all of these vaccines among children born in the preceding 3 years. The information obtained in the survey on differences in vaccination coverage among subgroups of children is considered useful for programme planning and targeting resources towards areas most in need. According to the guidelines developed by the World Health Organization, children are considered to have received all basic vaccinations when they have received a vaccination against tuberculosis (also known as BCG), three doses each of the DPT-HepB-Hib (also called pentavalent) and polio vaccines, and a vaccination against measles.
The BCG vaccine is usually given at birth or at first clinical contact, while the DPT-HepB-Hib and polio vaccines are given at approximately age 6, 10, and 14 weeks. Measles vaccinations should be given at or soon after age 9 months. The Malawi immunisation programme considers a child to be fully vaccinated if the child has received all basic vaccinations, three doses of the PCV vaccine (also given at age 6, 10, and 14 weeks), and two doses of the rotavirus vaccine (given at age 6 and 10 weeks). There are no vaccination programmes in place for older children.
At present children age 12-23 months are the youngest cohort to have reached the age by which a child should be fully immunised. 76% of children age 12-23 months received all basic vaccinations, and 71% are fully vaccinated. Only 2% of children had not received any vaccinations. Ninety-eight percent of children received the BCG vaccination, 98% the first dose of DPT-HepB-Hib, 97% the first dose of polio, 96% the first dose of the pneumococcal vaccine, and 96% the first dose of rotavirus vaccine. Ninety-one percent of children have received a measles vaccination.
Coverage rates decline for subsequent doses, with 93% of children receiving the recommended three doses of DPT-HepB-Hib, 81% the three doses of polio, 89% the three doses of the pneumococcal vaccine, and 91% the two doses of the rotavirus vaccine. There is little difference in the coverage rates between male and female children. Immunisation coverage increases with increasing mother’s education; three-quarters of children whose mothers have a secondary education are fully immunised (76%), as compared with 67% of children whose mothers have no education. Coverage is higher in rural than urban areas (72 and 66%, respectively).
The hepatitis B virus (HBV) vaccination programs offer a model for HPV introduction in which newborn and infant immunization achieves a rapid reduction in the prevalence of the HBV carrier rates and of liver cirrhosis and liver cancer decades later.
FIGIJ is now developing a worldwide PAG education strategy and supports preventive HPV prophylactic vaccination triggered by the knowledge (Van Damme et al) that the safety period obtained by the implementation is as long as >30 years.
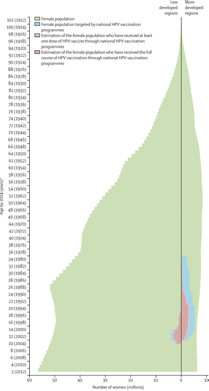 Left: Female population pyramid by development level and age distribution of women targeted by national HPV vaccination programmes
Left: Female population pyramid by development level and age distribution of women targeted by national HPV vaccination programmes
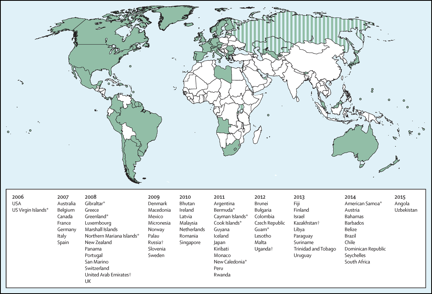 Right: We grouped countries with the UN classification system. More developed regions comprise Europe, northern America, Australia and New Zealand, and Japan. Countries that have introduced a publicly funded national human papillomavirus vaccination programme since 2006, by year (Bruni et al, 2016). Striped sections indicate implementation in a part of the country. French Polynesia, Liechtenstein, and Niue have reported vaccine programmes, but no information was available about year of introduction.
Right: We grouped countries with the UN classification system. More developed regions comprise Europe, northern America, Australia and New Zealand, and Japan. Countries that have introduced a publicly funded national human papillomavirus vaccination programme since 2006, by year (Bruni et al, 2016). Striped sections indicate implementation in a part of the country. French Polynesia, Liechtenstein, and Niue have reported vaccine programmes, but no information was available about year of introduction.
*Special territory. †Partial implementation. Less developed regions comprise all regions of Africa, Asia (except Japan), Latin America and the Caribbean, plus Melanesia, Micronesia, and Polynesia. HPV=human papillomavirus. *Birth cohorts of women are shown in parentheses.
Copyright © 2016 Bruni et al. Open Access article distributed under the terms of CC BY the Lancet global health 2016
The estimated full-course coverage of human papillomavirus vaccine by 2014, by age group and geographical region is represented on the map below.
 Copyright © 2016 Bruni et al. Open Access article distributed under the terms of CC BY the lancet global health 2016
Copyright © 2016 Bruni et al. Open Access article distributed under the terms of CC BY the lancet global health 2016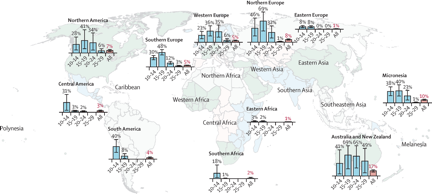
Error bars represent 95% CIs. Regions without bar charts had no or very low estimated coverage. More detailed data are provided in the appendix (pp 43–46).
Countries included in the analysis are geographically classified as follows: northern Africa (Libya); eastern Africa (Rwanda, Seychelles, Uganda); southern Africa (Lesotho, South Africa); Caribbean (Barbados, Cayman Islands, Dominican Republic, Trinidad and Tobago, US Virgin Islands); Central America (Mexico, Panama); South America (Argentina, Brazil, Chile, Colombia, Guyana, Paraguay, Peru, Suriname, Uruguay); northern America (Bermuda, Canada, Greenland, USA); central Asia (Kazakhstan); eastern Asia (Japan); southern Asia (Bhutan); southeastern Asia (Brunei, Malaysia, Singapore); western Asia (Israel, United Arab Emirates); eastern Europe (Bulgaria, Czech Republic, Romania, Russia); northern Europe (Denmark, Finland, Iceland, Ireland, Latvia, Norway, Sweden, UK); southern Europe (Gibraltar, Greece, Italy, Malta, Portugal, San Marino, Slovenia, Spain, Macedonia); western Europe (Austria, Belgium, France, Germany, Luxembourg, Monaco, Netherlands, Switzerland); Australia and New Zealand (Australia, New Zealand); Melanesia (Fiji, New Caledonia); Micronesia (Guam, Kiribati, Marshall Islands, Micronesia, Northern Mariana Islands, Palau); Polynesia (American Samoa, Cook Islands).
Copyright © 2016 Bruni et al. Open Access article distributed under the terms of CC By the Lancet global health 2016
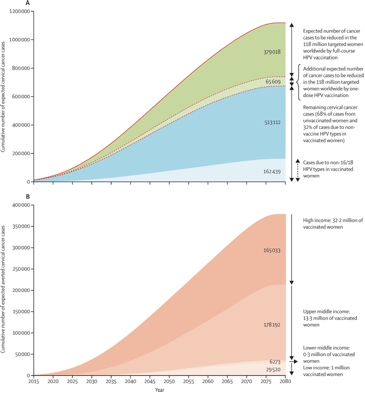
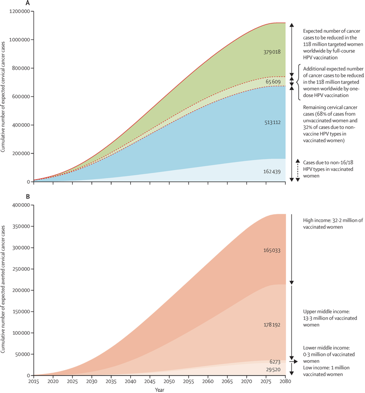
The estimated cervical cancer cases before age 75 years in the cohort of women targeted by HPV vaccination programmes by the end of 2014 are represented right.
(A) Estimated incidence of cervical cancer averted before age 75 years in the 118 million women ever targeted by HPV vaccination programmes. Solid line shows the cumulative number of expected cervical cancer cases up to age 74 years if targeted cohorts would not have been vaccinated. Dashed line shows the cumulative number of expected cervical cancer cases up to age 74 years in targeted cohorts considering current HPV vaccination coverage.
(B) Estimated incident cervical cancer averted before age 75 years in the 47 million fully vaccinated women by income level. Figure shows the cumulative number of expected cervical cancer cases up to age 75 years in vaccinated women assuming 70% vaccine effectiveness.
The Case for Early Gender Neutral Vaccination:
In a brave new world we should consider vaccinating much earlier, and even perhaps at birth. After all, the public health economy has to consider whether it is acceptable to allow young children and adolescents to succumb from a vaccine-preventable diseases.
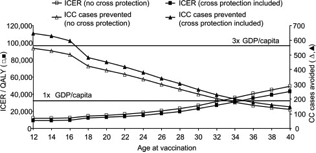 Impact of age at vaccination on ICER and number of cervical cancer cases avoided (with and without protection against non-HPV-16/18). CC, cervical cancer; GDP, gross domestic product (€32,200 for Belgium); ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year. This shows the projected incremental cost-effectiveness ratio (ICER) with and without cross protection, together with cost-effectiveness thresholds. Extending HPV vaccination to females post-sexual debut could lead to a substantial reduction in the cervical cancer-related burden and would be cost-effective in Belgium in women up to the age of 33-40 years (Demaerteau et al., 2013).
Impact of age at vaccination on ICER and number of cervical cancer cases avoided (with and without protection against non-HPV-16/18). CC, cervical cancer; GDP, gross domestic product (€32,200 for Belgium); ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year. This shows the projected incremental cost-effectiveness ratio (ICER) with and without cross protection, together with cost-effectiveness thresholds. Extending HPV vaccination to females post-sexual debut could lead to a substantial reduction in the cervical cancer-related burden and would be cost-effective in Belgium in women up to the age of 33-40 years (Demaerteau et al., 2013).Knowledge about early childhood HPV transmission warrants a different prevention policy and optimal timing of prophylactic vaccination (Kahn et al., 2002).When the vaccine came on the market, the focus was mainly the prevention of cervical cancer and girls and young women were the target population.
Nowadays gender neutrality in a vaccination one dose setting is to be considered.
This programme proposes the mass administration to children of both sexes of (preferably) the nonavalent one dose vaccine in a project based in designated African countries.
Currently, only a very limited number of boys have been vaccinated (Kim et al, 2007; Leon et al., 2008). Follow-up data from ongoing studies of HPV in boys and men lead to new vaccination strategies and changes recommendations. The initiative in childhood is completely up to the parents themselves. Gender inequity is unacceptable and in case of low coverage (<70%) herd vaccination (Gillison et al., 2010) is advisable
Data on the vaccination of boys have started to emerge. HPV vaccines are now approved for males in several countries, including Australia, South Korea, the United Kingdom, and the United States. Including boys in a vaccination programme is especially advisable if vaccine coverage of girls is less than 50% and when all other HPV-related disease is included (Cummings et al., 2012; Arbyn et al., 2012; Canfell et al., 2011). The quadrivalent vaccine has been shown to be effective in preventing genital warts and anal cancer in males. In 2009, the FDA licensed this vaccine in the United States for boys and men ages 9 to 26. Clinical trial data for the quadrivalent vaccine in 16- to 26-year-old males show it is well tolerated, induces a strong type-specific immunological response comparable to that of females, and reduces vaccine HPV-type-related genital infection, as well as disease. The data also suggest that vaccination of HIV-infected and other immunocompromised individuals may be of important value (Kahn et al., 2012).
Cost benefit analyses and mathematical modelling show that the most cost-effective strategy involves routine administration of this vaccine to 12-year-old females, with catch-up vaccination of 12- to 24-year-olds, and that the most effective strategy in terms of disease reduction would be to include men and/or boys in the programme. It is projected that by 2050, a vaccination strategy including 12-year-old boys would reduce HPV 16 infection by 88–94% in females and 68–82% in males, as well as reducing male HPV-related cancers by 22–27%. Therefore, the inclusion of males in an HPV vaccination programme is likely to bring significant health and economic benefits over and above those brought by current female-only programmes. However, comprehensive cost–benefit analyses are needed to determine the efficacy of these programmes in the overall population. Such analyses will be crucial for the design, acceptance, and implementation of these vaccination programmes into clinical practice globally (Garland, 2010).
The data on sexual abuse in children and the knowledge HPV is even at birth transmitted to underage children creates an innovative script for the child. In case of increased vulnerability like immunodeficiency and sexual experiences at young age, this young population has a real need for vaccination guidance. It has also been shown that positive chlamydia and other STI infection is frequently associated with HPV (Girardet et al, 2009). The acquisition of HPV is also mediated by bacterial vaginosis, which is very prevalent in adolescents (Syrjanen & Puranen, 2000).
Study results revealed not only an unexpectedly high frequency of HPV infections in underage girls, but also indicate that the genotyping profiles are somewhat aberrant from what is described for an adult population. The reasons for these differences are not well understood; potentially they might be explained by a hormonal influence that stimulates infection rates in different ways in each genotype (Mark et al., 2011; Bruni et al., 2010; Genco et al., 2004), supporting the role of a nonavalent vaccine.
The success of future HPV vaccination programs depends on the timing of vaccination, especially before and even after unplanned exposure. New multivalent HPV vaccines targeting a wider range of HR types obtain a better protection targeting HPV-6, 11, 16, 18, 31, 33, 45, 52, and 58 when administered before sexual debut but also after (Monsenego et al, 2012).
Significant political and advocacy efforts at the Global level need to be organised and reinforced to achieve a meaningful reduction in HPV transmission and its related benefit on health conditions and cancers.
There are obvious limitations for vaccination at a young age, none more so than vaccinations are currently not recommended for young children under the age of 9 or newborns.
However newer HPV prevention strategies may reduce even further the incidence of cancer. One example is the implementation of a nonavalent vaccine in the post exposure prophylaxis of sexual abuse as being a crucial way to control disease.
Large clinical datasets are proving the safety of two and three dose regimens in girls (using 2, 4 and 9 valent vaccines) and boys (4 and 9 valent vaccines) from the age of 9 years onwards.
Some case reports of 4 valent HPV vaccination experience in young boys under the age of 9 years with recurrent papillomatosis are confirming good immune responses (Lowy, 2016) and Gender neutrality in vaccination has been confirmed.
HPV immunological status in patients with recurrent respiratory papillomatosis (RRP) have shown low levels of seroreactivity against HPV 6 and/or HPV 11 despite many years of disease.
- Germany – 2-year-old boy with HPV 6 & 11-related RRP with CR after 4 valent HPV vaccination . Confirmed remission during 14 months of follow-up since vaccination. Good serological response (Förster et al, 2008)
- Hungary – 2-year-old boy – HPV 6 & 11-related RRP – 3 dose 4v HPV vaccine regimen – CR during 2y follow-up with excellent cell-mediated immune responses after HPV immunization, corresponding with remarkable clinical improvement (Mészner S et al. 2015).
- Netherlands – 6-year-old boy with HPV 6-related RRP. Good seroreactivity. (Tjon Pian Gi R et al, 2016)
- Czech Republic – 5-year-old girl HPV 11 related-RRP & frequent surgical interventions. CR after 4v HPV vaccination. Confirmed remission during 17 months of follow-up since vaccination. No immuno data. (Mudry P et al, 2011)
- Switzerland – 6-year-old-boy with dramatic HPV 6-related RRP with tracheostomy. Almost CR after 3 dose 4 v HPV vaccine regimen with tracheostomy closed at 5 months after the start of the treatment. No immunological data. (Xu, et al Int, 2014).
- In their 2010 IMPAACT/PACTG P1047 study Weinberg et al J looked at HIV+ children aged 7-12 years who tolerated well 3 doses of 4v HPV vaccine and generated robust and persistent cLIA antibodies to HPV6, 11, and 16 but comparatively weaker responses to HPV18. A fourth dose increased antibodies against all vaccine genotypes in an anamnestic fashion. Cytoxic T-Lymphocytes s and mucosal antibodies against vaccine genotypes, as well as cross-reactive antibodies and CTL against nonvaccine genotypes, were detected
Currently, in many countries women are vaccinated within the recommended 9 to 26 year age range, although the specific target age groups vary, depending on the country policy. The amenability of population vaccination policies to change depends on a variety of medical, financial, and socio-cultural factors. At this stage, no data are available to support the approval of vaccination of infants.
The term children will be used in this study to refer to persons under the age of 13 in our “to be vaccinated” population group. The broad definition of ‘child’ adopted here is consistent with the 1989 UN Convention on the Rights of the Child (CRC) in which the term ‘child’ refers to all human beings under the age of 18, unless the relevant national law recognizes an earlier age of majority.
In order to maintain momentum it is envisaged that mass campaigns every 5-12 years to administer a single dose of HPV vaccine to all children aged 5-12 years old.
Young women, especially before the age of 18, are considered to be a very vulnerable group. This can be explained by their sexual behaviour (casual sex, multiple partners, unsafe sex, etc.) and the immaturity of their genital tract, which can be vectors in the transmission of HPV (Stanley, 2010). In young adolescent (postpubertal) girls, the squamo-columnar junction is far out of the cervical canal and is at greater risk of HPV acquisition (in the prepubescent girl it is within the canal). The girl’s cervical ectopy and genital immunity are still immature and vulnerable to HPV and other infections, and one of the most common sexually transmitted infections affecting the cervix is the papilloma virus infection (Rocha-Zavaleta et al., 2004; Hwang et al., 2009).
The younger the postpubescent adolescent is at her first sexual intercourse, the more vulnerable her squamo-columnar junction is, which gives the virus an opportunity to infect metaplastic cells. Persistence of infection at the transformation zone of the cervix is key to the development of cervical cancer. If the immature immunity of the girl fails to eradicate the virus, a dysplasia can develop and, if not treated, eventually progress to cancer (Hager, 2009).
The correlation between early-age onset of sex and higher risk of HPV infection supports the need for public health messages about HPV vaccination, especially among low-income cohorts (Plummer et al., 2011; Burkett et al., 1992). An effective information campaign will undoubtedly determine the acceptance of early vaccination (De Gulglielmo, 2012).
When girls grow older and ovulatory cycles start, maturation of the defence mechanisms against infections follows. Also, other factors such as age, childbirth and invasive procedures tend to cause the transformation zone to move further up into the endocervical canal, where it is more protected from infection (WHO, 2004). Several other disease mediators have been described, including: socio-economic status, ethnic background, oral contraception, substance abuse (e.g. cannabis and tobacco smoking), circumcision, immunosuppression, sexual debut at a young age, open-mouth kissing, and malnutrition (Clifford et al., 2005; Clifford et al., 2006; D’Souza et al., 2009; Luhn et al., 2013).
Sexual practices other than vaginal intercourse, such as skin-to-skin contact, oral and anal sex are also potential infection pathways. This might explain the general impression that the use of condoms does not provide 100% protection against transmission of the virus. Consistent use of condoms reduces but does not eliminate the risk of HPV infection since HPV can still be transmitted through contact with unprotected genital skin (Koutsky et al., 2002; Manhart et al., 2002). There is data that genital HPV may also be a risk factor for increased risk of becoming HPV positive.
Oral transmission from mothers and fathers carrying one or more HPV types and vertical transmission during birth have been described (Lacour and Trimble, 2012). However, it remains a matter of debate whether mother-to-child transmission of HPV is important and whether these children are at higher risk.
Specific behavioural factors such as sexual intercourse at a young age have important implications for clinical practice and for the design of adolescent cancer-prevention programmes. Depending on the various factors influencing early transmission and infection, the risk to develop a genital cancer may be present at a much earlier age. The prognosis for children infected at and during the prenatal and perinatal period is not known. Knowledge about early childhood HPV transmission is also important to inform prevention policy and the optimal timing of prophylactic vaccination (Kahn et al., 2002).
As recently as 2015 the sexual initiation of children in Malawi has been highlighted in the context of “hyenas” – men who are paid to have sex with young girls as young as 10-11 years, unaware of the what is to happen and blindfolded, with only the elders knowing the identity of the man. No protection was used and some girls became pregnant.
Because child sexual-assault survivors are at increased risk for future unsafe sexual practices that have been linked to higher risk of HPV acquisition and are more likely to engage in these behaviours at an early age, the Centers for Disease Prevention and Control have issued Guidelines recommending vaccination of children who are victims of sexual abuse or assault at over the age of 9 years who have not initiated or completed immunisation. The Guidelines accept that whereas HPV vaccine will not protect against progression of infection already acquired or promote clearance of the infection, the vaccine protects against vaccine types not yet acquired.
The prevalence of HPV in minors can be deduced from the presence of HPV in a wide range of pathologies found in children, such as genital warts and respiratory papillomatosis.
In modelling costs and outcomes, HPV vaccination should be evaluated not only for its efficacy, but also from an economic point of view.
Programmes can be quickly undermined by rumours and misinformation if the reasons for targeting girls only are not fully and sensitively communicated. Educating men, including fathers and boys, about HPV vaccines and cervical cancer is particularly important in this regard. Providing cervical cancer information to older women and mothers of the girls being offered vaccination is a potential way to involve parents.
Focus on promotion is needed as disbelief and lack of knowledge leads to mistrust and low uptake. Convenient delivery platforms must be chosen, school based vaccination remains a good option
Informed consent for HPV vaccination can provide another communication opportunity to educate parents and children about adolescent health issues or cervical cancer screening (WHO, 2013).
Vaccines
New multivalent HPV vaccines targeting a wider range of High Risk types of HPV obtain better protection.
Gardasil 9 is starting to be integrated in vaccination programs.
- The primary immunogenicity objectives of non-inferiority of GMTs induced by GARDASIL 9 at 1 month after the second dose were met for the comparisons of girls and of boys, 9 to 14 years of age, who received either the 2-dose (0, 6) regimen or the 2-dose (0, 12) regimen with young women, 16 to 26 years of age, who received the 3‑dose (0, 2, 6) regimen.
- Based on these results, and using the same approach as that previously accepted for licensure of a 2-dose regimen of Gardasil, efficacy findings in young women who received the 3‑dose regimen of GARDASIL 9 can be extended to girls and boys 9 to 14 years of age who received the 2-dose regimen.
- GARDASIL 9 was generally well tolerated in all vaccination groups. There were no vaccine-related serious AEs (SAEs). Discontinuations due to an adverse event were rare (<0.1%).
Overall, the safety profile in this study did not reveal any new findings compared with previous studies in the GARDASIL 9 clinical program.
The new generation of multivalent vaccines targeting HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 may provide more benefit when administered before sexual debut (Monsenego et al., 2012). Most detected HPV infections are transient and not expected to cause high-grade cervical disease. On a global scale, vaccination of newborns and infants is well established and has developed a successful working infrastructure.
A model for HPV introduction is provided by the hepatitis B virus (HBV) vaccination programmes, in which newborn and infant immunization achieves a rapid reduction in the prevalence of the HBV carrier rates in immunized cohorts of children, and of liver cirrhosis and liver cancer decades later. Significant political and advocacy efforts at the global level (World Health Organization, other United Nations agencies and The GAVI Alliance) need to be organized and reinforced to achieve a meaningful reduction in HPV transmission and its related health conditions and cancers. The coordination of multidisciplinary approaches is necessary and plays a key role in the coordination of all players (health authorities, general physician, paediatrician scientific societies, social agents, media, parental uptake, etc) in order to achieve and maximize coverage rates.
Many environmental factors, including culture and poverty, can undermine vaccine uptake;, and decision-making about whether public health agencies can provide HPV prevention and cover the cost of the vaccine and the operational cost of its delivery is critical in low and middle income countries.
Improving the wellbeing of the young generation is essential. In order to minimize the burden, the risks and the psychological stress caused by a diagnosed HPV infection, we need to develop strategies for further prevention of the disease through patient-friendly vaccination programmes.
A multi-year process resulted in the consensus that regulatory agencies could consider additional endpoints, such as HPV protection, depending on the design of the HPV vaccine trials. Updating the guidelines will now accelerate vaccine coverage and complementary expertise will lead to regulatory approval.
The Case for Single Dose Vaccination
There is increasing evidence that one dose of bivalent HPV vaccine may be adequate to prevent most cases of cervical cancer (Kreimer et al, 2015). By June 2015 Mayor was advocating in the BMJ how one dose of HPV vaccine in preventing most cervical cancers. Brotherton also in 2015 commented that if HPV vaccines could be delivered as one dose, while retaining their efficacy against the most oncogenic HPV types, the global burden of cervical cancer would decrease substantially. Data from studies have shown how effective one vaccine dose can be in even the most resource poor settings, such as meningitis A vaccines in sub Saharan Africa.
The vaccine efficacy in young women receiving one dose of vaccine was 85.7% (95% confidence interval 70.7 to 93.7) compared with 77% (74.7 to 79.1) in those receiving three doses of vaccine after an average follow-up of four years. The difference in rates of protection between one and three doses was not statistically significant. The data indicates that one dose of bivalent HPV vaccine might be adequate to protect against HPV-16 and HPV-18 persistent infections and therefore probably disease. In this respect it is noteworthy that HPV-16 and HPV-18 cause more that 70% of cervical cancers and the vast majority of HPV-related cancers at other anatomical sites. Bivalent vaccines appear to impact on the rate of genital HPV.
More recent publications (Sankaranarayanan et al, 2016, Basu et al, 2016) support the concept of one dose of quadrivalent vaccine being as effective as or three in preventing persistent HPV 16/18 infection four years post-vaccination. The authors conclude that the short term protection affirded by one dose of HPV vaccine against persistent infection with HPV 16, 18, 6 and 11 is similar to that afforded by two or three doses of vaccine and merits further assessment.
This project offers an opportunity for partners to engage in global research opportunities in partnership with ImaGYNations including the WHO allowing for an opportunity to review their Guidelines on HPV vaccination.
Logistics/Implementation:
Charity base
Central co-ordination of programme
Acknowledgement that none of the available vaccines prevent cervical cancer completely
Ensure vaccines licensed in participating countries
Agreement on contracts
Vaccine supply and stocks
Regulations
Free school-based programme of administration
Possible combination with other established vaccination programmes
Informed consent
Audit and monitoring of the programme
References
Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, Gillison M, Bruni L, Ronco G, Wentzensen N, Brotherton J, Qiao YL, Denny L, Bornstein J, Abramowitz L, Giuliano A, Tommasino M, Monsonego J. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer 2012;131(9):1969-1982.
Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R, Peto J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30 Suppl 5:F88-99.
Basu P, Bhatia N, Ngoma T, Sankaranarayanan R. Less than 3 doses of the HPV vaccine – Review of efficacy against virological and disease end points. Hum Vaccin Immunother 2016; 12(6):1394-402
Brotherton JML. Could one dose of bivalent HPV vaccine prevent cervical cancer? 2015. Lancet Oncol doi:10.1016/S1470-2045(15)70253-6
Bruni Let al, Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis,Dr Laia Bruni, MD, et al, The Lancet Global Health Volume 4, Issue 7, Pages e453-e463 (July 2016)
Burkett BJ, Peterson CM, Birch LM, Brennan C, Nuckols ML, Ward BE, Crum CP. The relationship between contraceptives, sexual practices, and cervical human papillomavirus infection among a college population. J Clin Epidemiol 1992;45(11):1295-302.
Canfell K, Shi JF, Lew JB, Walker R, Zhao FH, Simonella L, Chen JF, Legood R, Smith MA, Nickson C, Qiao YL. Prevention of cervical cancer in rural China: evaluation of HPV vaccination and primary HPV screening strategies. Vaccine 2011;29(13):2487-2494.
Centers for Disease Prevention and Control. STD Treatment Guidelines. 2015
Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, Molano M, Rajkumar R, Ronco G, de Sanjosé S, Shin HR, Sukvirach S, Thomas JO, Tunsakul S, Meijer CJ, Franceschi S; IARC HPV Prevalence Surveys Study Group. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005;366 (9490):991-998.
Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006;24 Suppl 3:S3/26–34.
Cummings T, Zimet GD, Brown D, Tu W, Yang Z, Fortenberry JD, Shew ML. Reduction of HPV infections through vaccination among at-risk urban adolescents. Vaccine 2012;30(37):5496-5499.
De Sanjose, W.G. Quint, L. Alemany L, Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study, Lancet Oncol. 11 (2010) 1048–1056.
Demarteau N, Van Kriekinge G, Simon P.Incremental cost-effectiveness evaluation of vaccinating girls against cervical cancer pre and post-sexual debut in Belgium. Vaccine 2013;31:3962-3971.
De Guglielmo Cróquer Z. Development of Vaccines and Gene Therapy against HPV Infection and Cervical Cancer. Intech Press. From bench to bedside, a clinical perspective. D. Vanden Broeck 2012;177-189.
D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviours associated with prevalent oral human papillomavirus infection. J Infectious Dis 2009;199:1263-1269.
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.1 Cancer incidence and mortality worldwide: IARC CancerBase No 11. International Agency for Research on Cancer; 2014.
Forster G, Boltze C.,Seidel J.,Pawlita M, Muller A. Juvenile Larynxpapillomatose – Impfung mit dem polyvalenten Spaltimpfstoff Gardasil® Laryngo-Rhino-Otol. 2008,87: 796-799
Garland SM. Prevention strategies against human papillomavirus in males. Gynecol Oncol 2010;117: S20-S25.
C, Yuenger J, Zenilman J, Giles J, Ghanem K, Agarwal S, Ghanem KG. Sex hormones In uence the Types and Amount of Cytokines Produced by Human Papilloma Virus (HPV) Transformed Vaginal and Cer- vical Epithelial Cells. Abstract October 2004 IDSA Conference idsa.confex.com/idsa/2004/webprogram/ Paper19308.html.
Gillison M L, Chaturvedi A K, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervi- cal cancers in both men and women. Cancer 2008;113:3036-3046.
Girardet RG, Lahoti S, Howard LA, Fajman NN, Sawyer MK, Driebe EM, Lee F, Sautter RL, Greenwald E, Beck- Sagué CM, Hammerschlag MR, Black CM. Epidemiology of sexually transmitted infections in suspected child victims of sexual assault. Pediatrics 2009;124:79-86.
Hager WD. Human papillomavirus infection and prevention in the adolescent population. J Pediatr Adolesc Gynecol 2009;22(4):197-204.
Hwang L. Factors that influence the rate of epithelial maturation in the cervix of healthy young women, J Adolesc Health 2009, 44(2);103-110.
Joura EA, Guiliano AR , Iversen OE, Bouchard C, Mao C, Mehlsen J, et al., For the broad spectrum HPV study, N. Engl. J. Med. 372 (2015) 711–723.
Kahn JA, Xu J, Zimet GD, Liu N, Gonin R, Dillard ME, Squires K; Adolescent Trials Network for HIV/AIDS Inter- ventions. Risk perceptions after human papillomavirus vaccination in HIV-infected adolescents and young adult women. J Adolesc Health 2012;50(5):464-470.
Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost effectiveness analysis in a low-resource setting. Br J Cancer 2007;97:1322-1328.
Kreimer AR, Struyf F, Del Rosario-Raymundo MR et al Efficacy of fewer than three doses of an PPV-16/18 AS04 adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol 2015, doi:10.1016/S1470-2045(15)70199-3
Lazcano-Ponce E, Stanley M, Muñoz N, Torres L, Cruz-Valdez A, Salmerón J, Rojas R, Herrero R, Hernández- Ávila M. Overcoming barriers to HPV vaccination: Non-inferiority of antibody response to human papillo- mavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months Vaccine 2014;32(6):725-732.
Lacour DE, Trimble C. Human Papillomavirus in infants: transmission, prevalence, and persistence. J Pediatr Adolesc Gynecol. 2012;25(2):93-97.
Lowy DR1, Schiller JT ,Prophylactic human papillomavirus vaccines. J Clin Invest. 2006 May;116(5):1167-73.
Lowy D R HPV vaccination to prevent cervical cancer and other HPV-associated disease: from basic science to effective interventions, J Clin Invest. 2016;126(1):5–11.
Lowy DR, Herrero R, Hildesheim A, et al., Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immu- nobridging, Lancet Oncol. 16 (2015) e226–e233.
Luhn P, Walker J, Schiffman M, Zuna RE, Dunn ST, Gold MA, Smith K, Mathews C, Allen
RA, Zhang R, Wang S, Wentzensen N. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol 2013;128:265–270.
Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis. Sex Transm Dis 2002;29(11):725-735.
Mark M, Gravitt PE, Gupta SB, Liaw KL, Kim E, Phongnarisorn C, Wootipoom V, Yuenyao P, Vipupinyo C, Rug- pao S, Sriplienchan Tadesse AS, Celentano DD. The association of hormonal contraceptive use and HPV prevalence. Int J Cancer 2011;128(12):2962-2967.
Mayor S. One dose of HPV vaccine could prevent most cervical cancers, studies show. 2015. BMJ; 350:h3198 doi:10.1136/bmj.h3198.
Malavik ,et al Development of World Health Organization (WHO) recommendations for appropriate clinical trial endpoints for next-generation Human Papillomavirus , Papillomavirus Research 2 (2016) 185–189
Malawi Youth Data Sheet – 2014 – Population Reference Bureau
Meszner Zsofia, Jankovics Istvan, Nagy Aniko, Gerlinger Imke, Katona Gabor, Recurrent laryngeal papillomatosis with oesophageal involvement in a 2 year old boy: Successful treatment with the quadrivalent human papillomatosis vaccine,International Journal of Pediatric Otorhinolaryngology 79 (2015) 262–266
Mudry P, Martin V, Mazanek P, Machalova M, Litzman J, Sterba J ,Laryngeal papillomatosis: successful treatment with human papillomavirus vaccination, Arch Dis Child 2011;96:476–477. doi:10.1136/adc.2010.198184
Paavonen J, Naud P, Salmeron J, et al., Efficacy of human papillomavirus (HPV)- 16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women, Lancet 374 (2009) 301–314.
Plummer M, Peto J, Franceschi S. International Collaboration of Epidemiological Studies of Cervical Cancer. Time since first sexual intercourse and the risk of cervical cancer. Int J Cancer 2012;130(11):2638-2644.
Richmond AK, Priyanka S, Mahmood M et al, Paediatric & Adolescent Gynaecology in Europe: Clinical Services, Standards of Care and Training. J Pediatr Adolesc Gynecol, 2016; 29(3):299-303
Rocha-Zavaleta L, Yescas G, Cruz RM, Cruz-Talonia F. Human Papilloma virus infection and cervical ectopy. Int J Gynaecol Obstet 2004;85(3):259-266.
Sankaranarayanan R. Immunogenicity and HPV infection after one, two and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol 2016: 17(1):67-77 doi:10.1016/S1470-2045(15)00414-3.
Schiller JT, Muller M, Next generation prophylactic human papillomavirus vaccines, Lancet Oncol. 16 (2015) e217–e225.
Stanley M. Pathology and epidemiology of HPV infection in females. Gynaecol Oncol 2010;117:S5-10
Syrjanen S, Puranen M. Human papillomavirus infections in children: the potential role of maternal trans- mission. Crit Rev Oral Biol Med 2000;11(2):259-274.
E A Robin, P Tjon, R Michel, San Giorgi M, Pawlita M, MichelA, Bettien M. Ed van Hemel, E Schuuring, R E van den Heuvel4 • van der Laan B ,Dikkers F , Immunological response to quadrivalent HPV vaccine in treatment of recurrent respiratory papillomatosis Eur Arch Otorhinolaryngol 2016 DOI 10.1007/s00405-016-4085-3
Van Damme P, et al. Use of the nonavalent HPV vaccine in individuals previously fully or partially vaccinated with bivalent or quadrivalent HPV vaccines. Vaccine (2015), http://dx.doi.org/10.1016/j.vaccine.2015.12.063
Humoral, Mucosal, and Cell-Mediated Immunity Against Vaccine and Nonvaccine Genotypes After Administration of Quadrivalent Human Papillomavirus Vaccine to HIV-Infected Children Weinberg A, Song Lin-Ye, Saah A, Brown Martha, Moscicki A, Meyer W , Bryan J, Levin M, Journal of Infectious Diseases 2012;206:1309–18
World Health Organization, Human papillomavirus vaccines WHO position paper, WHO Wkly. Epidemiol. Rec. 15 (2009) 118–131 〈http://www.who.int/wer/〉 (2009/wer8415.pdf?ua=1, (accessed 15.05.15).
World Health Organization, Recommendations to assure the quality, safety, and efficacy of recombinant human papillomavirus virus-like particle vaccines. Replacement of: TRS 962,Annex〈http://www.who.int/biologicals/HPV_PostECBS_ZHOU_(CLEAN)_2810〉pdf, (accessed 06.02.16), 2015.
World Health Organization. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus-like particle vaccines. WHO Technical Report Series, Annex 1. 〈http://whqlibdoc.who.int/trs/WHO_TRS_962_eng.pdf?Ua=1〉, 2006 (accessed 15.05.15).
World Health Organization. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus-like particle vaccines. WHO Technical Report Series, Annex 1. 〈http://whqlibdoc.who.int/trs/WHO_TRS_962_eng.pdf?Ua=1〉, 2006 (accessed 15.05.15).
Xu Y, Liu S, Yi H, Wang J, Dong P, Li X, et al. (2014) Human Papillomavirus Infection in 674 Chinese Patients with Laryngeal Squamous Cell Carcinoma. PLoS ONE 9(12): e115914. doi:10.1371/journal.pone.0115914
